Pb-free perovskite for solar is stable in air
Author: EIS Release Date: Jul 30, 2019
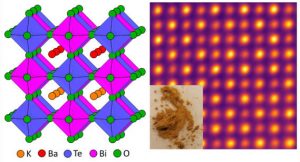
US researchers have used a supercomputer to discover a candidate for Pb-free perovskite solar cells – most solar perovskites contain Pb-halides, and are unstable in moist air.
KBaTeBiO6 is the material, a bismuth-halide double perovskite that was the most promising of 30,000 candidate bismuth-containing oxides postulated by a team of engineers at Washington University in St Louis using data analytics and high-throughput quantum-mechanical density functional theory calculations – of the 30,000, only ~25 were already known, according to the university.
“We found that this looked to be the most stable compound and that it could be synthesised in the lab,” said researcher Rohan Mishra. “Whereas most oxides tend to have a large band, we predicted the new compound to have a lower band gap, which is close to the halide perovskites, and to have reasonably good properties.”
Shalinee Kavadiya, then at WASTL, now at Arizona State University, managed to make it using wet-chemistry after six months of work.
“Once she was able to synthesise it, as we had predicted, it was stable and had a band gap of 1.88eV, which we also predicted,” said Mishra, who described the discovery as a good first step toward non-toxic solar cells – pointing out that the most promising compounds for solar cells have a band gap of ~1.5eV.
The work is published as ‘KBaTeBiO6: A lead-free, inorganic double-perovskite semiconductor for photovoltaic applications‘ in Chemistry of Materials.
Only the abstract is available without paying – according to which: “Our work demonstrates the untapped potential of inorganic Bi-based double perovskite oxides – that offer the ability to change both the cation combination and their stoichiometry to achieve desired electronic properties – as exciting, benign, and stable alternatives to Pb-halide perovskites for various semiconducting applications.”
According to supplementary information published alongside the paper, x-ray diffraction analysis suggests that a sample of the powder has not substantially changed after 380 days of ambient storage.
Next, the team will study the role of any defects in this new semiconductor and look to more advanced synthesis techniques.
Diagram: A sketch of the atomic structure on the left, and a scanning transmission electron micrograph of it on the right, inset with a photo of the synthesised powder. Credit: Rohan Mishra.
